Author: R&D Team, CUIGUAI Flavoring
Published by: Guangdong Unique Flavor Co., Ltd.
Last Updated: Nov 1, 2025

Flavor Production Scaling
For manufacturers of food and beverage flavours, scaling production from the pilot phase to full commercial volume is a critical leap. The pilot might show success, but replicating that success at commercial scale—while maintaining flavor integrity, cost‐efficiency, regulatory compliance, production robustness, and supply chain control—requires a detailed, structured strategy. When you’re supplying aroma systems to large‐scale food & beverage producers, it’s not enough to have a strong flavour design; you also need to ensure that your manufacturing is prepared to scale effectively and reliably.
In this blog post, we provide a technical, authoritative, and well-structured guide to scaling up flavour production—from pilot design through to commercial launch and ongoing operations. We will focus on flavour manufacturers (like your company) supporting clients or your own internal scale‐up of aroma modules. We cover:
Throughout, we will include reference to relevant literature and industry insights to bolster credibility and signal to Google that this is a high-value technical resource.
When you design a flavour concentrate or aroma system, you often start in small batch labs or pilot tanks (tens to hundreds of litres). At that scale, things like mixing, dosing, ingredient behavior, stability, instrumentation, supply and logistics can be tightly controlled. However, when moving to commercial scale (thousands to tens of thousands of litres, drum/IBC scale, multiple shifts, higher through‐put, more operators, bigger mixing vessels, different heat/transfer dynamics, supply chain complexity) many phenomena change—and if they are not managed, flavour performance, cost or consistency suffer.
The article “Scaling food production: 12 critical dos and don’ts” by CRB Group highlights that ingredient behaviour, mixing, heat transfer and cleaning methods all change fundamentally when moving from small scale to large scale. Similarly, the review “Scaling up and out as a Pathway for Food System Transitions” shows that scaling operations in food systems pose unique technical, organisational and logistical risks. For flavour manufacturers, failure to scale properly can mean: inconsistent sensory profiles, increased re‐work or scrap, supply chain breakdown, regulatory non‐compliance, cost overruns and brand risk for your clients.
Therefore, mastering scale-up is not optional—it’s strategic. Whether your flavour modules are being moved into large batch production for your own plant, or you’re supporting a client’s manufacturing of finished consumer goods, having a robust scale-up framework means you deliver quality, consistency, cost and time reliability.
Scaling is not simply “multiply the batch size by 10 and use a 10× tank”. Many non‐linearities and new constraints emerge. Here are major challenge areas, and why flavour manufacturers must address them proactively.
When moving from a pilot (~100 L) to commercial (~5 000 L or more) mixing vessel, heat and mass transfer, shear, mixing kinetics, surface‐to‐volume ratio all change. For example, the article “8 Key Challenges to Pilot Plant Scale‐Up” outlines how fluid dynamics and reaction kinetics shift when scale increases. For flavour production, this means that aroma concentrate dissolution, mixing of flavour modules, homogeneity, and ingredient interaction may behave differently at scale. If mixing is slower, flavour dispersion may be incomplete. If heating/cooling is slower, thermal stress may affect flavour compounds. Equipment designed for pilot may not ensure identical behaviour in full scale.
At small scale you may use select high‐cost aroma ingredients, special extract batches, custom containers. At scale you may face sourcing constraints, cost pressures, supply variability. The CRB article emphasizes that scaling ingredient sourcing and delivery method is a key “don’t” if not considered early. As flavours scale, stability behaviour may change (e.g., oxidative losses, container headspace differences, agitation differences). Also, the sequence of addition, temperature profiles, shear may all modify flavour behaviour.
Equipment requirements at scale are more demanding—larger mixing tanks, pumps, piping, instrumentation, CIP systems, material handling for drums/IBCs. Facilities may need upgraded utilities (steam, cooling, compressed air, vacuum, etc). The heat transfer challenge means you may need different heating/cooling systems when scale increases. If you fail to adapt infrastructure, flavour quality or yield may suffer.
At commercial scale you must deliver consistent flavour quality across multiple batches, shifts, operators, possibly at multiple sites. The larger scale often magnifies variability (batch drift, operator error, supply variation). The concept of design space verification—ensuring that your process inputs and material inputs at scale can deliver expected quality—is critical. Without design space insight, small changes at scale may push you outside the reliable operating window.
Scaling means larger volumes of raw material, more complex logistics, storage, sequestering of space, packaging scale, inventory management, possibly global sourcing. The CRB article points out that ingredient sourcing at scale may require different vendors, delivery format, logistics. For your flavour manufacturing business, this means you must evaluate sustainability of supply, vendor capacity, cost pressures.
Large scale manufacturing often has stricter regulatory, hygiene, sanitation, CIP requirements. Change‐over times increase, cleaning between flavour variants becomes more complex. If not managed, risk of cross‐contamination, carry‐over or downtime increases.
Scaling introduces large capital and operating cost. Delays or inefficiencies at scale multiply cost. The article “From Pilots to Production: Scaling Sustainable Tech in Food Manufacturing” by Food Industry Executive shows that scaling must be disciplined and cost management is essential. For a flavour house, you must ensure your scale‐up strategy delivers acceptable economics and supports your clients’ cost targets.
Scaling production requires new skills in operators, maintenance, quality assurance. Pilot teams may be small and closely managed; commercial operations involve many operators, shifts, possibly multiple sites. Ensuring consistency of training and procedures is critical.
Understanding these challenges upfront allows you to build mitigation plans and avoid the “just scale up and hope” approach. The next section provides a systematic strategy.
Here we present a structured roadmap to scale up flavour production from pilot grade to commercial success. Each step is oriented from a flavour manufacturer’s perspective and oriented to food & beverage flavour modules.
Begin with establishing what “success” looks like at scale. Some example criteria:
Clear KPIs let you guide decisions and measure readiness.
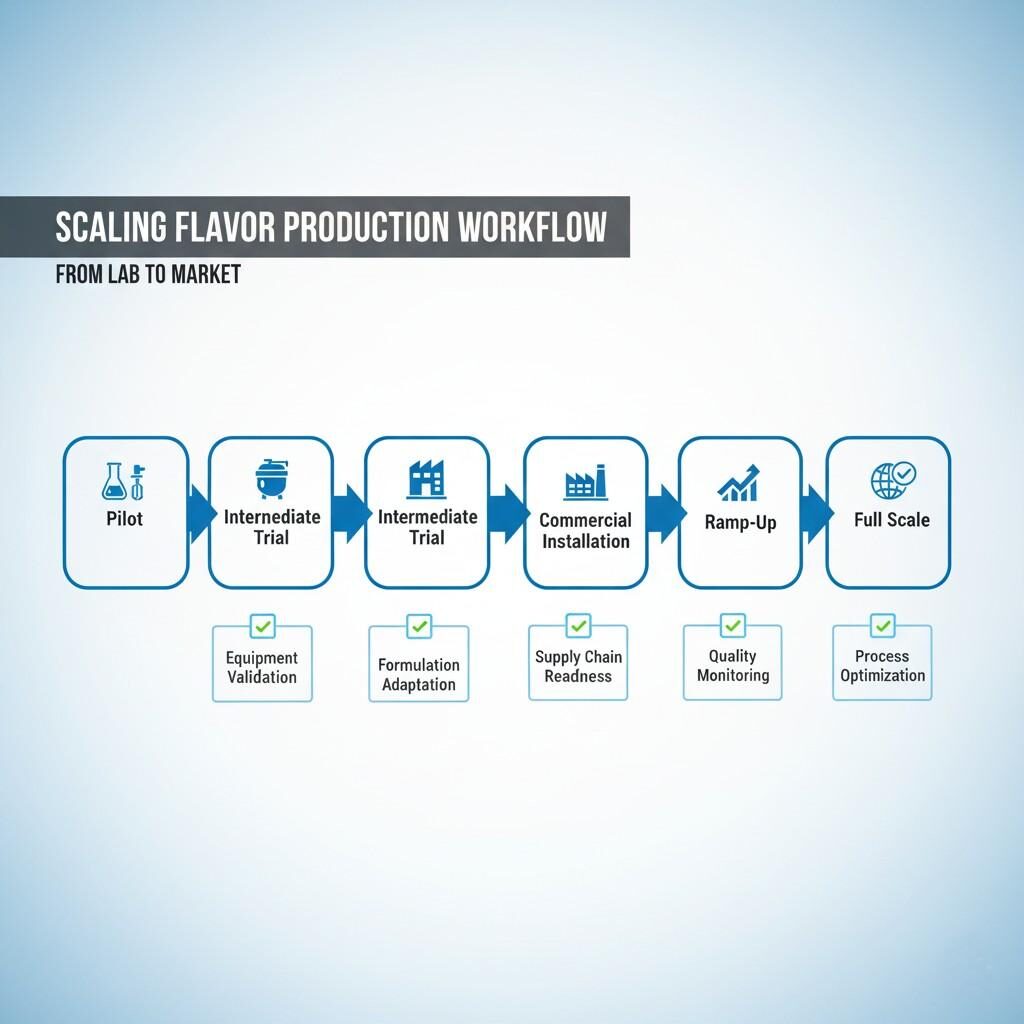
Flavor Production Scaling Workflow
Here are best practice guidelines focused on flavour manufacturers moving to commercial production:
Ensure R&D, process engineering, quality assurance, supply chain, production, and marketing are aligned early. Many scale‐up problems stem from siloed teams. Collaboration helps anticipate ingredient supply, regulatory impact, CAPEX/utility demands.
Create detailed flavour module specification sheets including:
Use structured risk assessment to identify scale‐up risks (ingredient behaviour, mixing, stability, contamination, CIP, change‐over). For each risk define mitigation, triggers and monitoring. The bioprocess scale-up review emphasises that pre‐production PHA is essential to avoid cost overruns.
Avoid jumping directly from small pilot to full commercial; use incremental scale steps such as 5× or 10× pilot. This gives opportunities to validate mixing, shear, heat transfer, ingredient behaviour, pumping, headspace and storage dynamics. Each intermediate step refines the process.
Define critical process parameters (CPPs) such as mixing time, temperature, shear, dosing accuracy, headspace oxygen, storage temperature. Define critical quality attributes (CQAs) like aroma strength, flavour profile, stability over time, physical appearance. Monitoring these helps ensure scale success.
At commercial scale, simply assuming “pilot taste” will hold is risky. Use analytical methods (e.g., GC‐MS, HPLC) and sensory panels to verify flavour modules at scale. Manage documentation and deviation procedures accordingly.
In flavour manufacturing, change-over between modules and variants is frequent. Ensure that CIP systems, purge times, verification checks are designed for large volumes. Neglecting this drives downtime and flavour carry‐over risk.
Larger scale means more wear, greater thermal load, and greater utility demand. Schedule maintenance, monitor utilities (steam, cooling water, vacuum), ensure redundancy. Consider energy efficiency too: as the “From Pilots to Production” article highlights, large scale gives opportunity for improved efficiency.
Ensure aroma concentrate raw materials are available at higher volumes, confirm vendor capacity, shipping and storage logistics (e.g., drums/IBCs vs 5 L pails). Review packaging scale, warehousing, backup suppliers, lead‐times and cost impact. The CRB article emphasises this is a “do or don’t” item.
Track cost drivers: ingredient cost per kg, yield losses, reject rate, energy cost, labour cost, change‐over downtime. Use lean manufacturing practices, SPC and OEE (Overall Equipment Efficiency) to improve yield, reduce waste and ensure COGS targets are met.
Once you are in full‐scale production, maintaining performance is just as important as achieving it. Here’s how your flavour manufacturing business supports ongoing success.
Maintain a dashboard of key indicators like:
When a deviation occurs (for example a flavour batch shows weaker aroma, or stability drop), use root‐cause frameworks to investigate. Verify batch records, equipment logs, ingredient lots, mixing times, cleaning records, storage conditions. Document corrective/preventive actions. Over time, refine your master specification and process.
Large scale provides volume advantage but also risk. Ensure multiple sources for critical aroma raw materials, monitor cost trends, adapt formulations if needed (with your R&D support) to maintain quality at acceptable cost. Consider hedging, long‐term contracts, forecasting.
Large scale operations often need flavour variant changes for market demands. Build flexibility into your manufacturing (modular tanks, quick‐changeover CIP, standardised dosing modules) so you can launch new variants without major downtime. Your flavour house should support clients here by offering scalable aroma modules designed for easy integration.
At scale, utility costs and environmental impact become significant. The “From Pilots to Production” article highlights how scaling up offers opportunity for efficiency improvements in utilities and process. As a flavour manufacturer you should include sustainability metrics (energy, water, waste), support your own manufacturing and your clients’ flavour application systems.
In commercial production, traceability and documentation become more critical. Maintain lot traceability for aroma modules, raw material batches, processing logs, cleaning records, sensory reports. Ensure change‐control protocols, validation and qualification records are current. This supports both quality assurance and regulatory readiness.
Large scale operations face greater risk from equipment failure, ingredient supply disruption, flavour drift, contamination, recall. Establish contingency plans: alternate suppliers, backup equipment, batch hold points, ready‐to‐execute corrective actions. Make your flavour house part of the contingency ecosystem.
As a professional manufacturer of food & beverage flavours, you play a pivotal role in helping your clients—or your own business—transition from pilot to commercial scale. Here are specific ways you deliver this value.
Your R&D should not only produce flavour modules for small batch tests, but design them with scale in mind: packaging (drums/IBCs), dosing compatibility, pumpability, stability under large batch conditions, homogeneity across large mix volumes. Include specification sheets, supply‐chain risk assessments, upstream process data.
Supply detailed documentation: typical batch records for flavour module production at scale, mixing guidelines, dosing recommendations, packaging/storage instructions, stability/shelf‐life data. Offer technical service to client production teams for commissioning, pilot trials, scale adaptation.
Offer training modules for client operators and your internal teams on scale‐up best practices: mixing behaviour, equipment considerations, dosing, CIP/change‐over, troubleshooting. Prepare your team and your clients for scale challenges upfront.
Maintain a product pipeline of flavour modules that are easily scalable and flexible, enabling variant launches at commercial volume. Provide modular dosing systems, quick‐change flavour portfolios, and assistance in ramp‐up for new flavour launches.
Ensure your own sourcing strategy is robust for large volumes of aroma raw materials. This stability enables you to supply clients at scale without disruption. Offer cost transparencies and strategies to manage increased volumes.
By acting not just as a supplier but as a partner in scaling your clients’ flavour production, you strengthen your strategic position. Offer consultations, pilot‐to‐commercial transition planning, joint risk assessment, ongoing improvement services.
Scaling up flavour production from pilot to commercial volume is a complex but manageable journey—provided you adopt a structured strategy, anticipate the non-linear changes, align cross‐functional teams, and build the right infrastructure, formulation, supply chain and monitoring systems. For flavour manufacturers and aroma system suppliers, success in scale means not just creating compelling flavour profiles but enabling their consistent, cost-effective, high‐volume production.
By establishing clear objectives, rigorous process engineering, robust formulation strategy, pilot validation, commissioning, KPI monitoring and continuous improvement, you deliver quality, efficiency and reliability at scale. As your business supports food & beverage brands in this journey, you provide not just aroma modules but strategic value—ensuring flavour integrity and compliance at commercial production.
If you’re ready to embark on scale-up of your flavour manufacturing—or support a client’s transition from pilot to high volume—now is the time to act.
Commercial Flavor Plant

Commercial Flavor Plant
Are you planning to scale your flavour production from pilot to commercial? We’d love to assist. Contact us for a technical exchange and request a free sample kit of our scale-ready aroma modules. Let’s partner to build your flavour manufacturing future—efficiently, reliably, and at commercial scale.
🌐 Website:[www.cuiguai.cn]
💬 Whatsapp:[+86 189 2926 7983]
📩 Email:[info@cuiguai.com]
📞 Phone: [+86 0769 8838 0789]
Copyright © 2025 Guangdong Unique Flavor Co., Ltd. All Rights Reserved.